PBE Expert Inc, we Process, Build, Engineer
Services d’experts-conseils
PBE Expert Inc, offre des services en ingénierie des procédés, validation, layout, efficacité énergétique et gestion de projet dans diverses industries.
PBE Expert Inc, we Process, Build, Engineer.
Votre partenaire en génie des procédés et en désinfection COVID, met à votre service une expertise de +25 ans sur +200 projets conformes.
Expert MAPAQ & Santé Canada.

Notre Equipe
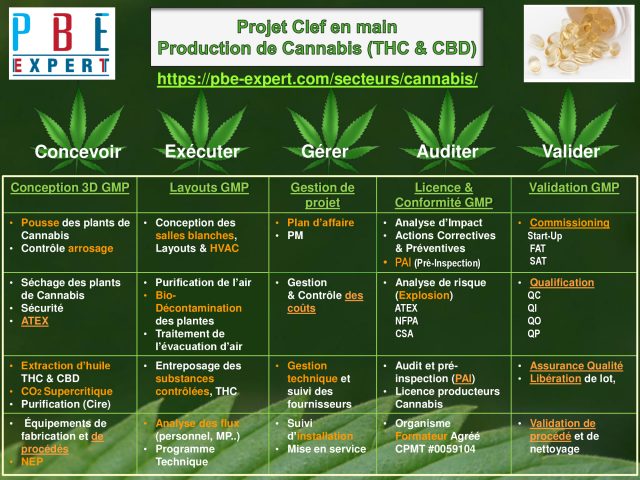
Vous recherchez un partenaire en ingénierie des procédés, utilités propres, gestion de projet, NEP, layout, conformité, validation, AQ, Audit PAI, calibration ou efficacité énergétique?
Actualité
Activités récentes
Novembre 2017
Étudiants Polytechnique chez PBE
ILS NOUS ONT FAIT CONFIANCE
Clients
Bureau
CANADA
Pour toute information commerciale, de partenariat et marketing avec PBE Expert Inc
Nous sommes aussi au :